ivig multiple sclerosis
Intravenous immunoglobulin IVIg August 2018 In the past immunoglobulins have been used as a disease modifying treatment for multiple sclerosis in the UK and they are still used in many other European countries. Glossary AE adverse event.
Multiple Sclerosis or MS affects the brain spinal cord and optic nerves.
. Multiple Sclerosis IVIg was once promising. This could be the case in women who are planning a pregnancy or in women who shouldnt discontinue therapy throughout pregnancy because of the course of the disease. Data on the utilization of IVIg in pregnancy are still limited.
In the Lancet in 1997. In den letzten Jahren wurden mehrere klinische Studien zur Wirksamkeit von IVIG bei MS veröffentlicht. View LargeDownload Cox proportional hazards model estimates of the cumulative probability of developing clinically definite multiple sclerosis.
A statistically significant difference between groups of P03 was reached after 8 months. Clinical evidence of the effects of IVIg in multiple. Systemic Sclerosis SSc is a rare autoimmune disease that is characterized by a progressive skin fibrosis an obliteration of the microvasculature and an exaggerated extracellular matrix deposition which lead to a multisystemic dysfunction.
Four double-blind trials have been performed of IVIG in relapsing-remitting MS. A randomized placebo-controlled trial of monthly IVIG in RR MS was reported by Fazekas et al. The promises and uncertainties of intravenous immunoglobulin IVIg therapy for relapsingremitting multiple sclerosis RRMS.
Although IV immunoglobulin IVIG treatment was well tolerated this study did not substantiate a beneficial effect of IVIG in doses ranging from 02 to 04 gkg. This result seriously questions the utility of IVIG for the treatment of relapsing-remitting multiple sclerosis. Intravenöse Immunglobuline IVIG werden off-label als Reservemedikament zur Behandlung der Multiplen Sklerose MS bei Kontraindikationen Unverträglichkeiten oder unzureichender Wirkung der etablierten Therapien eingesetzt.
Despite initial positive indications these treatments have been mostly disappointing. Although intravenous immunoglobulins IVIg have shown some therapeutic effects in multiple sclerosis reducing global supplies restriction of treatment to essential indications and availability of effective alternative treatments for MS currently exclude IVIg from being an accepted therapy for MS other than for some exceptional considerations. After all IVIG is the only so.
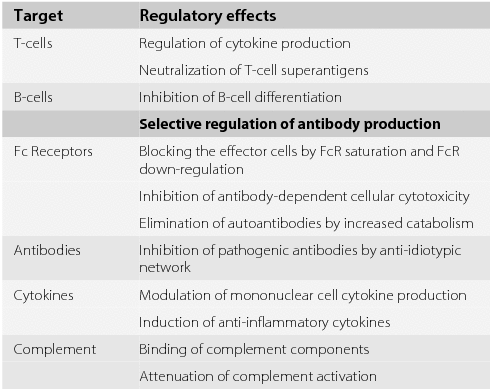
Reduction of inflammation inhibition of macrophages and promotion of remyelination. Modulation of the disease course by IVIg is achieved both by limiting the inflammatory process and by enhancing remyelination. For the most part the treatments for MS have evolved from immunosuppressive steroids and chemotherapy to the immunomodulators like Avonex Betaseron and Copaxone.
Some of that initial hope has faded. The rationales for IVIg therapy in RRMS are presented on the left happy face while the possible reasons for uncertainties with IVIg therapy are depicted on the right sad face. This can be given in the hospital or at the outpatient infusion center.
Despite promising clinical trials intravenous immunoglobulin IVIg therapy in relapsingremitting multiple sclerosis MS has met with uncertainties. Intravenous immunoglobulin IVIg pooled from healthy human volunteers has a role in several immunomodulating mechanisms which may affect the pathogenesis of multiple sclerosis. The usual dose for prevention of relapses maintenance therapy as used by most studies is 02 to 04 g kg every four weeks.
IVIG exerts a number of effects that may be beneficial in multiple sclerosis MS. Additionally IVIg so far seems to be the only possibility to enable sufficient breastfeeding periods of 6 months 40 with the potential to prevent relapses in the postpartum period of women with MS. Nevertheless IVIg treatment is a safe treatment option during the gestational period and.
Despite promising clinical trials intravenous immunoglobulin IVIg therapy in relapsingremitting multiple sclerosis MS has met with uncertainties that might be attributed to small patient cohorts heterogeneity in the patients. Various pathogenetic mechanisms were described. IVIg indicates intravenous immunoglobulin.
It is the result of the bodys immune system attacking a substance called myelin which wraps around nerve fibers to protect them. The usual IVIG dose for treatment of acute relapses is 04 gkgday for five days given as continuous IV infusion. A potential treatment effect ofIVIg in MS could be mediated by several mecha-nisms as IVIg is able to modulate the.
This is where multiple. For patients with relapsing-remitting multiple sclerosis intravenous immunglobulines can represent an alternative to other first line therapies. Initial positive clinical trials.
This placebo-controlled trial involved 150 patients over. However use of IVIG has been supplanted by the availability of FDA-approved disease modifying medications Beta-interferons and glatiramer acetate for the treatment of relapsing-remitting multiple sclerosis. Without myelin exposed nerves can become damaged often forming scar tissue which interferes with nervous system functions.
Potential effects andmethodologyofclinical trials PerSoelberg Sorensen Abstract Thereis extensive evidencethatimmune mechanisms are involved in the patho-genesis of multiple sclerosis MS. The lack of a successful therapy make SSc a disease with a poor prognosis. Baseline Characteristics View LargeDownload Table 2.
Ivig For Ms An Overview Of Ivig Benefits For Treatment Of Multiple Sclerosis
Intravenous Immunoglobulin To Treat Multiple Sclerosis Chapter 38 Multiple Sclerosis Therapeutics

Ivig For Relapsing Remitting Multiple Sclerosis Promises And Uncertainties Trends In Pharmacological Sciences

Intravenous Immunoglobulin To Treat Multiple Sclerosis Chapter 38 Multiple Sclerosis Therapeutics

Beneficial Effects Of Intravenous Immunoglobulin As An Add On Therapy To Azathioprine For Nmo Igg Seropositive Neuromyelitis Optica Spectrum Disorders Multiple Sclerosis And Related Disorders
Intravenous Immunoglobulin For Relapsing Remitting Multiple Sclerosis Treatment A Case Report And Literature Review

Intravenous Immunoglobulin Promotes The Proliferation Of Cd4 Cd25 Foxp3 Regulatory T Cells And The Cytokines Secretion In Patients With Guillain Barre Syndrome In Vitro Sciencedirect

The Mechanisms Of Action Of Ivig On Various Arms Of Autoimmune And Download Scientific Diagram

Altus Biologics What S Intravenous Immunoglobulin And How Can Help M S

Polyclonal Immunoglobulin G For Autoimmune Demyelinating Nervous System Disorders Trends In Pharmacological Sciences

Modulation Of The Cellular Immune System By Intravenous Immunoglobulin Trends In Immunology

Pdf Differentiating Characteristics And Evaluating Intravenous And Subcutaneous Immunoglobulin Semantic Scholar

Schematic Representation Of Proposed Mechanisms Of Action Of Ivig In Download Scientific Diagram

The Impact Of Ivig On The Innate And The Adaptive Immune Compartments Download Scientific Diagram

Intravenous Immunoglobulin To Treat Multiple Sclerosis Chapter 38 Multiple Sclerosis Therapeutics

Intravenous Immunoglobulin To Treat Multiple Sclerosis Chapter 38 Multiple Sclerosis Therapeutics

Successful Intravenous Immunoglobulin Treatment In Relapsing Mog Antibody Associated Disease Multiple Sclerosis And Related Disorders

Intravenous Immunoglobulin Ivig Induce A Protective Phenotype In Microglia Preventing Neuronal Cell Death In Ischaemic Stroke Springermedizin De

Intravenous Immunoglobulin As Clinical Immune Modulating Therapy Abstract Europe Pmc



0 Response to "ivig multiple sclerosis"
Post a Comment